Nankai’s Team designed New Materials for Sodium-Ion Batteries
2016.12.06
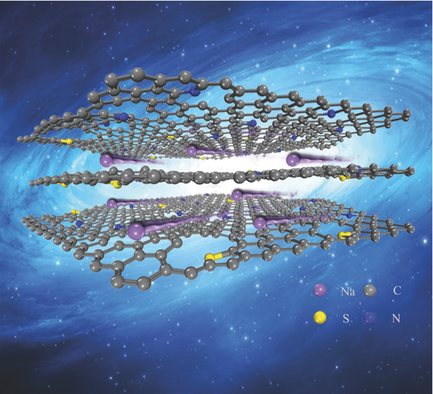
Recently, Zhou Zhen, Professor at Nankai University’s School of Materials Science and Engineering, and his team has provided a real breakthrough in the research on new materials for sodium-ion batteries. The team produced a new S-Doped N-Rich Carbon Nanosheets and calculated its mode of action. The results of the research were published in “Advanced Materials”, a scientific journal covering Materials Science.
As the most widely used commercial technology, lithium-ion batteries (LIBs) have been the key devices in energy storage and supply because of their high energy density. However, the insufficient quantity of lithium and the high cost inhabit the large-scale applications of LIBs to electric vehicles and smart grids. To solve the problem, Prof. Zhou Zhen and his team applied a simple and controllable method, achieving the replacement of specific N atoms by S atoms in the N-rich carbon nanosheets, produced by sol–gel approach. Thus, interlayer distance becomes large enough for Na+ insertion and diffusion. The large surface area and stable structure also provide more sites for Na+ absorption, leading to high Na- storage capacity and excellent rate performance. Moreover, Faradaic reactions between Na+ and tightly bound S is beneficial for further improvement of Na-storage capacity.
Moreover, Prof. Zhou and his team calculated that the cycling performance of S-N/C and N/C electrodes was evaluated by galvanostatic charge–discharge measurements at a current density of 50 mA g−1, and S-N/C exhibits much higher capacities than those of N/C, which kept steady for 200 cycles (Figure 3c). After slow fading in the initial 20 cycles, S-N/C cells could deliver stable reversible capacities of 350 mAh g−1 with nearly 100% Coulombic efficiency. The capacity is much higher than that of N/S-codoped carbon materials already reported. When the current density is turned back to 50 mA g−1, the reversible capacity is recovered up to ≈350 mAh g−1. To further elucidate the remarkable Na-storage performance at high current densities, after the test of rate performance, the cell was evaluated at the current density of 1 A g−1 for long-term cycling.Therefore, the excellent rate performance, once again, indicates the stable structure of electrode materials for S-N/C with expanded interlayer distance and large surface area.
Prof. Zhou explained that, in recent years, thanks to the rapid development of smart mobile electronic devices and electric vehicles, the large and constant consumption of lithium caused a dramatic increase in its price. Taking lithium carbon as an example, in 2016 alone, its price increased by three times, about 128 thousand RMB/ton. The high raw material cost largely decreased the possibility of developing large-scale storage devices, so discovering new storage technologies is very important. Compared to other materials, sodium-ion batteries has rich resources (for example, the price of natrium carbonicum, also known as soda, is about 1,000 RMB/ton), high energy-transforming efficiency, and long cycling life. Thus, it became a hot topic in the research on storage technology and products. Besides, developing low-cost electrode materials with an excellent electrochemical performance is one of the key factors to achieve the practical application of sodium-ion batteries.
The research is supported by NSFC, MOST in China, and Golone-Nankai Joint Laboratory for New Materials Computational Centre for Molecular Science.
Link to the paper: http://onlinelibrary.wiley.com/doi/10.1002/adma.201604108/full