Research on Autophagy Vesica Transportation and Regulation of Nankai University’s Team made Progress
2016.12.20
In recent years, more and more research showed that autophagy, as a self-protective mechanism for collective organization, can adapt to various environments and stimulations to keep the normal physiological function of the engine body. Therefore, autophagy is vital to the inner stability and survival of the engine body. Deficiency of autophagy could also cause many diseases, like Parkinson’s disease, diabetes (II), some gerontal diseases and even cancer. The research on the mechanism of autophagy became a hot topic in the field of Cytobiology. A research of Nankai University’s team led by College of Life Sciences’ Prof. Chen Quan and Prof. Zhu Yushan revealed the regulation of mATG9 trafficking by Src- and ULK1-mediated phosphorylation in basal and starvation-induced autophagy.
Autophagy is a cell survival process in which portions of the cytosol and damaged or unwanted organelles are engulfed into a double-membrane autophagosome and delivered to the lysosomes for degradation and recycling. Under normal physiological conditions, autophagy is maintained at a basal homeostatic level, which is responsible for protein quality control and turnover of intracellular organelles.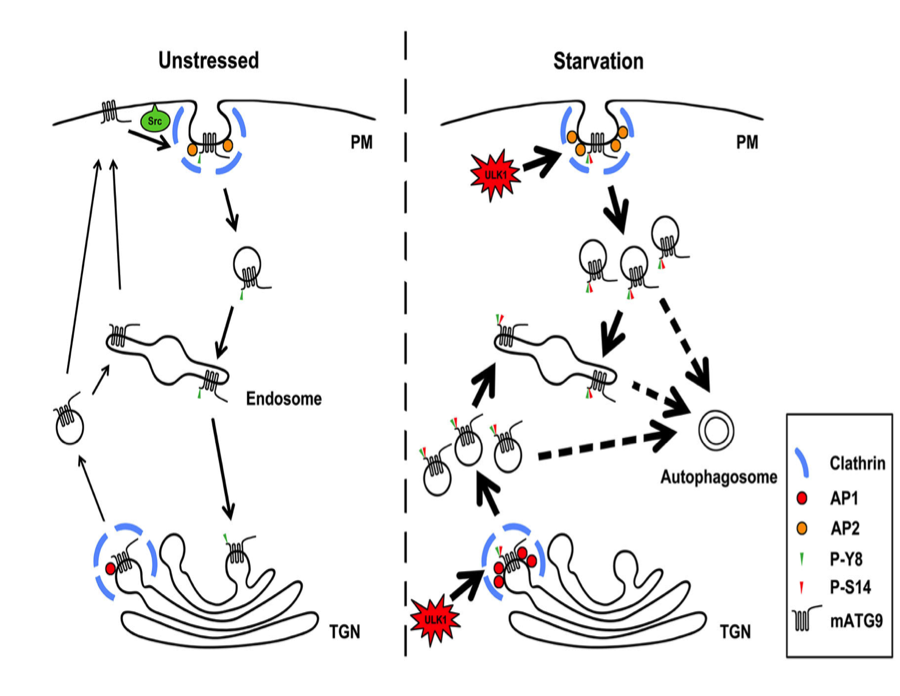
Regulation of Src and ULK1 kinase phosphorylation on mATG9 vesicular transportation
Autophagy requires diverse membrane sources and involves membrane trafficking of mATG9, the only membrane protein in the ATG family.
Nankai University’s team discovered and confirmed two conserved classic adaptor protein sorting signals within the cytosolic N-terminus of mATG9, which mediate trafficking of mATG9 from the plasma membrane and trans-Golgi network (TGN) via interaction with the AP1/2 complex. Src phosphorylates mATG9 at Tyr8 to maintain its endocytic and constitutive trafficking in unstressed conditions. In response to starvation, phosphorylation of mATG9 at Tyr8 by Src and at Ser14 by ULK1 functionally cooperate to promote interactions between mATG9 and the AP1/2 complex, leading to redistribution of mATG9 from the plasma membrane and juxtanuclear region to the peripheral pool for autophagy initiation. Their findings uncover novel mechanisms of mATG9 trafficking and suggest a coordination of basal and stress-induced autophagy.
The paper was published online in “Cell Research”, an international famous academic journal, on December 9. Zhou Changqian, a Ph.D. student, is the first author of the paper, and Prof. Chen Quan and Prof. Zhu Yushan are the corresponding authors. The working unit of the first author is the State Key Laboratory of Medicinal Chemical Biology. The research is supported by National Natural Science Foundation.
The team also cooperated with Prof. Xia Bin from Peking University, analyzing the structural basis for the phosphorylation of FUNDC1 LIR as a molecular switch of mitophagy. A related paper was published in “Autophagy”, an international journal. Ma Kaili, a Ph.D. student at Nankai University, and Kuang Yao, Ph.D. student at Peking University, are the first authors.
Just like apoptosis and cell senescence, autophagy is a very important biological phenomenon, as it participates in the biological growth, development, and other processes. In response to nutrient deprivation or other sub-lethal stresses, autophagy is geared up to a high flux to efficiently degrade and recycle cytoplasmic components for homeostasis and cell survival. Defects in autophagy have been causally linked with degenerative, inflammatory, metabolic and neoplastic diseases.
The researchers noticed the mechanism of autophagy more than 50 years ago, but its core importance in physiology and medical science was realized in the 90s when the progressive research of the Japanese cell biologist Yoshinori Ohsumi was published. Yoshinori Ohsumi won 2016 Nobel Prize in Physiology or Medicine for his discoveries of mechanisms for autophagy.
Links to the papers:
http://www.nature.com/cr/journal/vaop/ncurrent/full/cr2016146a.html
http://www.tandfonline.com/doi/abs/10.1080/15548627.2016.1238552?journalCode=kaup20